Practical Guidance for MedTech Reimbursement and Market Access
Insight on what matters most, and what it all means for your next milestone.
Why ASC-Heavy Technologies Might Do Well to Launch in the Hospital Setting First
Learn why ASC-heavy medical technologies may benefit from launching in hospitals first, from shaping Medicare payment rates to building evidence and adoption momentum. ...more
Payer Coverage ,Commercialization
November 24, 2025•5 min read

Medicare Advantage: What MedTech Leaders Must Know
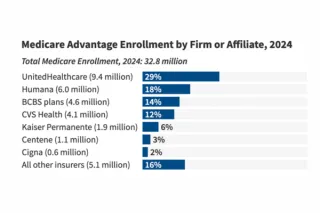
Understand how Medicare Advantage plans make coverage and payment decisions, and why MA strategy is essential for MedTech targeting Medicare-aged populations. ...more
Payer Coverage ,Commercialization
November 24, 2025•4 min read
When Reimbursement Doesn’t Match MedTech Value
Explore why coding and payment pathways often fall short of a MedTech company’s value story and how to navigate codes, bundling, rates, and payment gaps. ...more
Coding and Payment ,Payer Coverage
November 24, 2025•5 min read
Quantifying Value for Niche Medtech in a Payer Conversation
Learn how niche MedTech innovators can quantify value, link outcomes to claims data, and build payer trust through clear, localized evidence. ...more
Payer Coverage ,Commercialization
November 19, 2025•4 min read

How MedTech Innovators Can Win Payer Coverage Outside Heart Failure, Cancer, and Diabetes
Think your device won’t get payer traction because it’s outside the “big three”? This article breaks down how MedTech companies targeting niche patient populations can still win with U.S. payers by al... ...more
Payer Coverage
November 16, 2025•3 min read

Featured Insights for MedTech Innovators
Insights to help you validate your pathway, align with payer expectations, and move forward with confidence.

Clinical Trial Reimbursement in the U.S.: FAQs for MedTech Companies
A clear FAQ guide on how MedTech startups can secure Medicare reimbursement for clinical trials—and when it’s worth it. ...more
Coding and Payment ,Evidence Planning &Featured
March 19, 2025•4 min read

Beware Basing Your MedTech Pricing on Short-term New Tech Payment Mechanisms
New tech payments like NTAP can’t justify long-term pricing. Here’s what MedTech founders need to know. ...more
Coding and Payment ,Commercialization &Featured
March 04, 2025•3 min read

The Hidden Bias in Reimbursement: Why First Movers May Have It Easier
Why do better technologies struggle for reimbursement while outdated ones thrive? Discover the hidden bias in payer decision-making—and why second movers in MedTech face steeper evidence hurdles than ... ...more
Coding and Payment ,Evidence Planning Fundamentals &Featured
February 06, 2025•4 min read

The World After FDA Clearance: From HOORAY! To WAIT, WHAT?
FDA clearance is a milestone—not the finish line. Why reimbursement hurdles follow right after. ...more
Commercialization ,Fundamentals &Featured
August 20, 2024•5 min read

How Hospital Financial Decisions Shape the Fate of New MedTech Innovations
Hospitals are the gatekeepers of device adoption. Learn how to align with their economics. ...more
Commercialization ,Fundamentals &Featured
August 07, 2024•5 min read

Relearning English When it Comes to FDA and CMS Jargon
MedTech teams often misunderstand what regulators mean. This article translates FDA and CMS jargon into actionable insights for reimbursement. ...more
Fundamentals ,Featured
June 04, 2024•1 min read
© 2026 Coustier Advisory LLC. All rights reserved.
Powered by Rocket Boost
